 Freshwater mussels (also known as unionids) and their shells, have been used throughout history for a variety of different purposes. Native Americans used the shells as tools, but probably only ate the mussels as a last resort due to their foul taste and contaminants that accumulate in the mussels’ bodies. In the early 1900s, unionids were overharvested for the pearl button industry which led to a steep decline in population numbers. Today, it is illegal to collect live unionids or shells without a permit in Michigan, but freshwater mussels are still sent overseas to fuel the cultured pearl industry in Asia (Michigan Natural Features Inventory 2003).
Freshwater mussels (also known as unionids) and their shells, have been used throughout history for a variety of different purposes. Native Americans used the shells as tools, but probably only ate the mussels as a last resort due to their foul taste and contaminants that accumulate in the mussels’ bodies. In the early 1900s, unionids were overharvested for the pearl button industry which led to a steep decline in population numbers. Today, it is illegal to collect live unionids or shells without a permit in Michigan, but freshwater mussels are still sent overseas to fuel the cultured pearl industry in Asia (Michigan Natural Features Inventory 2003).
Freshwater mussels are bivalves, which means that they have hinged shells that enclose a soft body. 44 species of unionid are found in Michigan, living in rivers, streams, ponds, and lakes throughout the state. 19 of the 44 species are endangered or threatened and 12 are of special concern (Hanshue et al. 2019).
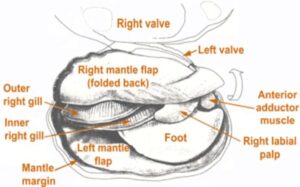 Unionids have relatively long lifespans; many species can live for 20 to 30 years with some species having been found to live for 60 to over 100 years (Great Plains Nature Center 2017) The shell is made of calcium carbonate and protein which creates a hard, protective barrier that can remain intact for years after death (Tennessee Wildlife Resource Agency, n.d.).
Unionids have relatively long lifespans; many species can live for 20 to 30 years with some species having been found to live for 60 to over 100 years (Great Plains Nature Center 2017) The shell is made of calcium carbonate and protein which creates a hard, protective barrier that can remain intact for years after death (Tennessee Wildlife Resource Agency, n.d.).
Life Cycle
Male unionids release sperm into the water. The female siphons in the sperm which travel to the female’s gills where the sperm fertilize the eggs (Winhold 2004). The gills are where the embryos grow and develop before they are ready to be released into the water. 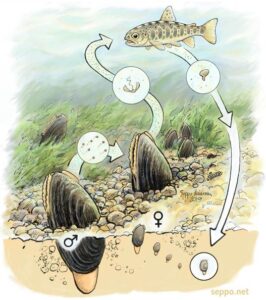 An important and unique aspect of the unionid life cycle is that the mussel larva, called glochidia, require a host fish to attach to (A species found in Michigan (but not in the Kalamazoo Watershed), the Salamander Mussel, is unique in that it uses the mudpuppy instead of a fish as a host) (Badra, n.d.). Some species of unionids are specialists, which means that they only have a few suitable host fish species, while other species are generalists, and thus have a wide range of host species they can use (Modesto et al. 2017). If the glochidia attach to the wrong host fish species, it will die from the host fish’s immune system response. Unionids have multiple strategies to make sure their offspring find and attach to a host fish. One strategy is for the female to just release the glochidia into the water and hope that they come into contact with a suitable host fish. This is risky because there is a high probability that the glochidia will not come into contact with a suitable host fish in time and without a suitable host fish the glochidia will die (Winhold 2004). This is why many species have evolved tactics that increase the odds that the glochidia will attach to a suitable host quickly. Some species use lures that are specialized mantle flaps that appear to look like small prey fish.
An important and unique aspect of the unionid life cycle is that the mussel larva, called glochidia, require a host fish to attach to (A species found in Michigan (but not in the Kalamazoo Watershed), the Salamander Mussel, is unique in that it uses the mudpuppy instead of a fish as a host) (Badra, n.d.). Some species of unionids are specialists, which means that they only have a few suitable host fish species, while other species are generalists, and thus have a wide range of host species they can use (Modesto et al. 2017). If the glochidia attach to the wrong host fish species, it will die from the host fish’s immune system response. Unionids have multiple strategies to make sure their offspring find and attach to a host fish. One strategy is for the female to just release the glochidia into the water and hope that they come into contact with a suitable host fish. This is risky because there is a high probability that the glochidia will not come into contact with a suitable host fish in time and without a suitable host fish the glochidia will die (Winhold 2004). This is why many species have evolved tactics that increase the odds that the glochidia will attach to a suitable host quickly. Some species use lures that are specialized mantle flaps that appear to look like small prey fish.

These fish lures attract the host fish looking for a meal. When the host fish attacks the lure, the female releases her glochidia which then attach onto the fish’s gills. Another way unionids can attract host fish is by creating sacks of glochidia, called conglutinates, that look like worms which the female either reels out into the water or releases for the conglutinate to attach to a rock.
When a host fish tries to eat the conglutinate, the sac bursts, releasing the glochidia which then attach to the host (Warren 2006). There is also the host capture strategy where the female uses her shell and adaptor muscles to clamp down on the fish and then releases her glochidia (MichiganDNR 2020).
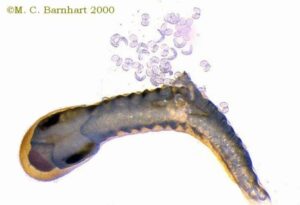
These are just a few examples of the diversity of glochidia release strategies used by mussel species.
Depending on the species, female unionids can produce anywhere from 2,000 to 10,000 glochidia at one time! The survival rate for the glochidia is very low with 99.99% of glochidia failing to find a suitable host (Modesto et al. 2017).
Once the glochidia come into contact with the host fish, they clamp onto its gills or fins. At this stage, the glochidia only contain adaptor muscles that hold the shell together. For 18-60 days, depending on the species, the parasitic glochidia develop and metamorphose on the host fish, taking in nutrients from the fish’s blood without harming it. Once the glochidia metamorphose into fully developed juvenile mussels, they release their hold on the host fish and drift down to the sediment. Mussels have a foot that they can use to burrow and anchor themselves in sediment and travel short distances, but for the most part, mussels live a sessile, or immobile, life (Michigan Natural Features Inventory 2003).
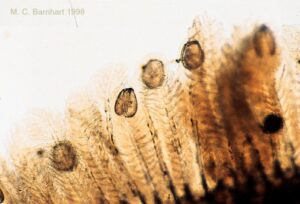 The main benefit of a host fish is that they aid in the dispersal of unionid populations. Host fish can carry mussels far from their parent which distributes populations across a wider range.
The main benefit of a host fish is that they aid in the dispersal of unionid populations. Host fish can carry mussels far from their parent which distributes populations across a wider range.
Importance
Unionids play a critical role in improving water quality. In addition to nutrients and oxygen, unionids also siphon in toxins and contaminants that get stored in the mussel’s body (U.S. Fish & Wildlife Service, n.d.). Unionids are sensitive to these contaminants and thus are good indicators of water quality (Michigan Natural Features Inventory 2003). Finding many live, healthy mussels in an area indicates that the water quality is good. Studies have found that water filtration by unionids is more effective than water filtration plants and can significantly increase water clarity as well as change the particle content in the water (Michigan Natural Features Inventory 2003). A study at Central Michigan University found that the unionid density found in Kalamazoo can filter 108 gallons of water every hour and 2600 gallons every day.

That means that every day, unionids in the Kalamazoo River are filtering the equivalent of 65 bathtubs of water!
Not only are unionid filtering capabilities impressive, their shells are used in a variety of different ways. Unionid shells not only provide a protective barrier for their bodies, but also provide a protective barrier for other organisms. The shells can last for years after death, and empty shells can be used by other organisms for shelter or as a protected location to lay their eggs. When conducting research, empty shells can be used to determine community composition or for chemical analysis (Michigan Natural Features Inventory 2003).
Unionids play an important part in the food web of Michigan lakes and rivers. They have the highest percentage of biomass compared to other benthic stream organisms (Michigan Natural Features Inventory 2003). Unionids have many predators such as muskrats, mink, raccoons, birds, fish, and river otters which rely on unionid populations for food (Badra, n.d.; U.S. Fish & Wildlife Service, n.d.). Unionids are filter feeders that eat algae, diatoms, and bacteria (Winhold 2004).
Threats
Negative Impacts
Unionid population decline is being driven by human impact on the environment. Point and non-point pollution can harm unionid health and even lead to death. Point pollution is pollution that enters the environment from one place such as discharge pipes, and non-point pollution is pollution that is released in a wide area such as pollutants that get transported by runoff (National Geographic, n.d.).  Unionids in Kalamazoo are affected by non-point pollution such as runoff that contains pesticides, herbicides, and fertilizers as well as point pollution such as PCB contamination from paper mills and oil from the 2010 Kalamazoo Oil Spill (Michigan Department of Environmental Quality. 2016; U.S. Fish & Wildlife Service, n.d.). The oil released from Enbridge Energy Partners Pipes released an estimated one million gallons of oil into the Kalamazoo River (Badra 2010). Both PCBs and oil can be found in the sediment of the Kalamazoo River where unionids live (Michigan Department of Environmental Quality. 2014). Pollution also affects unionids indirectly by negatively affecting the host fish necessary for the Unionid life cycle. Heavy metals can disrupt the immune and reproductive systems of fish and PCBs can cause deformities, reproductive issues, and even death in fish (Michigan Natural Features Inventory 2003; Modesto et al. 2017). To avoid impacted areas, fish might move to more habitable areas. Unionids live stationary lives and thus cannot move with their host fish species which they require for their life cycle and dispersal (Modesto et al 2017).
Unionids in Kalamazoo are affected by non-point pollution such as runoff that contains pesticides, herbicides, and fertilizers as well as point pollution such as PCB contamination from paper mills and oil from the 2010 Kalamazoo Oil Spill (Michigan Department of Environmental Quality. 2016; U.S. Fish & Wildlife Service, n.d.). The oil released from Enbridge Energy Partners Pipes released an estimated one million gallons of oil into the Kalamazoo River (Badra 2010). Both PCBs and oil can be found in the sediment of the Kalamazoo River where unionids live (Michigan Department of Environmental Quality. 2014). Pollution also affects unionids indirectly by negatively affecting the host fish necessary for the Unionid life cycle. Heavy metals can disrupt the immune and reproductive systems of fish and PCBs can cause deformities, reproductive issues, and even death in fish (Michigan Natural Features Inventory 2003; Modesto et al. 2017). To avoid impacted areas, fish might move to more habitable areas. Unionids live stationary lives and thus cannot move with their host fish species which they require for their life cycle and dispersal (Modesto et al 2017).
Dispersal can also be hindered by habitat loss and human made barriers. This includes, but is not limited to, roads, dams, stream channelization, and water abstraction (Hanshue et al. 2019). When dispersal is restricted for both the host fish and mussels, small isolated populations form. Small populations reduce the genetic diversity which reduces population growth rate, decreases the population’s ability to adapt to environmental changes, and decreases disease resistance in the population. All of these factors make it difficult for small isolated populations to survive and reproduce leading to local extinctions which can eventually lead to the extinction of the entire species (Perdue University., n.d.).
Invasive Species
Zebra Mussels (Dreissena polymorpha) are native to the Black, Caspian, and Azov Seas and arrived in the United States in the early 1980s transported in ship ballast water. Their preferred habitat is inland lakes but can also be found in rivers. One female Zebra mussel can produce one million eggs per year and their larva do not require a host fish for dispersal. Instead the larva are free floating and travel with the water current (State of Michigan, n.d.).

Like Native freshwater mussels, Zebra mussels are filter feeders that eat phytoplankton, zooplankton, and algae which leads to competition for food resources between the native and invasive mussels. One adult zebra mussel can filter one liter of water per day which leads to Zebra mussels outcompeting Native mussels for food and a significant decrease in phytoplankton and zooplankton populations (Tip of the Mitt Watershed Council, n.d.). They also bioaccumulate contaminants which can travel up the food chain as predators eat the mussels (Tip of the Mitt Watershed Council, n.d.). After only 2-3 years Zebra mussels die and all the contaminants stored in their bodies are released back into the water, causing water quality issues Unfortunately, one contaminant that Zebra mussels are not as good at filtering out compared to native mussels is e coli, creating a health hazard for humans (Thorp 2018).
In addition to food competition, Zebra mussels are a problem because they attach to hard surfaces and are difficult to remove. This includes native mussel shells which is why native mussel populations that live on softer substrate might be at a higher risk of infestation (Michigan Natural Features Inventory 2003). When zebra mussels attach to native mussel shells in large numbers, they can restrict movement, block siphons, create shell deformities, and deposit metabolic wastes onto the native mussel, all of which restrict native mussels from reproducing and feeding. Zebra mussels also attach to pipes, boats, water equipment, and docks, blocking pipes, deteriorating docks and steel, and sinking buoys (State of Michigan, n.d.).
 Another invasive species found in Michigan is the Asian Clam (Corbicula fluminea). The species is native to Asia, Africa, and Australia and was introduced to the United States in 1938 as a source of food (National Invasive Species Information Center, n.d.). Its preferred habitat is lakes or streams with sand or gravel substrate (Naumann 1999). Asian clams are hermaphrodites which means they have both male and female reproductive sex organs. They have the ability to self-fertilize and in one year can release 70,000 juveniles. In large densities the Asian clam can clog and damage waterways and pipes, with the cost of repair nationwide being $1 billion annually (Golden Sands Resource Conservation & Development Council, n.d.). Like the Zebra mussel, Asian Clams are filter feeders that compete with native mussel species for food and, in large densities, might consume native mussel juveniles, glochidia, and sperm (Michigan Natural Features Inventory 2003).
Another invasive species found in Michigan is the Asian Clam (Corbicula fluminea). The species is native to Asia, Africa, and Australia and was introduced to the United States in 1938 as a source of food (National Invasive Species Information Center, n.d.). Its preferred habitat is lakes or streams with sand or gravel substrate (Naumann 1999). Asian clams are hermaphrodites which means they have both male and female reproductive sex organs. They have the ability to self-fertilize and in one year can release 70,000 juveniles. In large densities the Asian clam can clog and damage waterways and pipes, with the cost of repair nationwide being $1 billion annually (Golden Sands Resource Conservation & Development Council, n.d.). Like the Zebra mussel, Asian Clams are filter feeders that compete with native mussel species for food and, in large densities, might consume native mussel juveniles, glochidia, and sperm (Michigan Natural Features Inventory 2003).
Freshwater mussels need your help to stop the spread of invasive species that threaten their populations. To help stop the spread, it is important to clean boats and water equipment after every use. This includes removing all drain plugs from bilges, ballast tanks, and live wells, removing aquatic plants, animals, and debris, and power washing your boat (Michigan Invasive Species 2020). Remember that just because you cannot see an adult mussel on your boat or equipment, this does not mean that the free-floating larva are not hitching a ride to your next water destination!
Research and Surveys

Dr. Woolnough’s lab at Central Michigan University’s Institute for Great Lakes Research is leading the way in freshwater mussel research. In 2018-2019, Dr. Woolnough’s research team conducted a mussel survey in the Kalamazoo River where they looked at 191 sites, 122 of which were surveyed. 69 of the sites were not surveyed due to either logistical reasons or the sites were determined to be bad habitats for mussels. Some logistical challenges of the survey included asking landowner permission to conduct the survey on their property and deep-water levels. In low water levels, surveying can be conducted through hand grubbing and using viewing buckets. At medium water levels, researchers combine the use of snorkeling with hand grubbing, and for deep areas, the team had to scuba dive (Hanshue et al. 2019). The deep sites along the main branch of the Kalamazoo River were conducted in 2019. 22 species of live mussels were found with a total of 3,416 live unionids. All shells were collected, and 4 more species were found as only shells.

The most common species found were the Plain Pocketbook, Spike, and Fat mucket. The species Lilliput is state endangered and the Slippershell is state threatened. Zebra Mussels were found at 8 sites downstream of lakes (Zebra mussels prefer lakes but are transported downstream by boats), and Asian clams were found in the main branches of the Kalamazoo river. In addition to unionid population counts, the team also collected data on water quality in 2019-2020. This data included water conductivity, turbidity, chlorophyll, and temperature as well as chemical concentrations of ammonia, phosphorus, calcium, and magnesium. This research was funded by a generous grant from the Kalamazoo River Recreational Foundation.
In addition to research, Dr, Woolnough’s lab was involved with outreach events to educate the community on information about freshwater mussels.

The team has a mobile lab in the form of a trailer that also acts as a moving classroom (Piatek 2019). The trailer contains shelves with tanks of live mussels and shells to show the community what different species look like. The trailer also has a table with microscopes so that guests can observe the microscopic glochidia with their own eyes. There are also activities for guests to participate in such as children looking for shells in sandboxes which teaches the children techniques that researchers use to survey mussels in sediment. The main goal of these outreach events is to teach communities about mussels’ roles in an ecosystem and why it is important to protect water systems from pollutants that can negatively affect mussels.
Another interesting new program that Central Michigan University is taking part in is the propagation facility at the Michigan DNR Saline Research Hatchery.

The goal of this project is to come up with ways to propagate, or breed, mussels to help increase populations. This is the first mussel propagation program in the state of Michigan. Funding for this project also comes from the grant from the Kalamazoo River Recreational Foundation. Depending on the age class of the mussels, they are kept in either dog pans, raceways, or buckets; with the dog pans and raceways used for juveniles, and buckets can hold 10s of juveniles and adults. These propagation efforts can also be used to quarantine mussels and protect them after a disaster such as an oil spill.
One specific species that the team is working on is the Snuffbox mussel, an endangered species. The project for this species is being conducted at Webber Dam in Ionia county with the help of the Michigan Department of Natural Resources, U.S. Fish and Wildlife Service, Ionia Conservation District, and Consumers Energy (Johnston 2020). The snuffbox mussel requires the host fish species, the Logperch, for its life cycle. The strategy that snuffbox mussels use to help their glochidia find and attach to a host fish is the clamp strategy. When a logperch swims down to the sediment to investigate the snuffbox mussel, the mussel traps the fish between its shells and pumps out its glochidia which attach to the Logperch’s gills.

For the propagation project, this part of the life cycle occurs in aqua filters in a trailer. The dam where this propagation project is taking place has a fish ladder that allows fish species to travel from one side of the dam to the other during their migration to spawning grounds. Once the Logperch are inhabited by snuffbox glochidia, they are transferred to cages which are placed in the fish ladder. The Logperch and glochidia will remain in these cages for the next 16 months as the glochidia grow and metamorphose (MichiganDNR 2020). The goal of this project is to eventually release the snuffbox mussels into the Grand River to boost their populations (Johnston 2020).
References
Badra, Pete. n.d. “Freshwater Mussels of Michigan”. Accessed September 17, 2020. https://mnfi.anr.msu.edu/pdfs/FreshwaterMusselsOfMichigan.pdf.
Badra, Pete. 2010. “Mussel Shell Survey Report: Kalamazoo River Unionid Mussel Shell Survey in the Marshall and Battle Creek Area”. Accessed September 25, 2020. https://www.fws.gov/midwest/es/ec/nrda/MichiganEnbridge/pdf/EnbridgeNRDADraftDARP_EAMay2015AppendixI.pdf.
Barnhart, M. C. 2008. “Unio Gallery”. Accessed November 6, 2020. http://unionid.missouristate.edu.
Golden Sands Resource Conservation & Development Council. n.d. “Aquatic Invasive Species Quick Guide Asian Clam”. Accessed October 30, 2020. https://www.uwsp.edu/cnr-ap/UWEXLakes/Documents/programs/CLMN/AISfactsheets/01AsianClam.pdf.
Great Plains Nature Center. 2017. “Unionid Mussels in Kansas”. Accessed November 13, 2020. https://gpnc.org/mussels/#:~:text=Mussels%20may%20live%20over%20100,extinct%20or%20in%20serious%20decline.
Hanshue, Scott et al. 2019. “Michigan Freshwater Mussel Survey Protocols and Relocation Procedures”. Accessed September 17, 2020. https://www.fws.gov/midwest/eastlansing/te/pdf/MI_Freshwater_Mussel_Survey_Protocol.pdf.
Illinois State Museum. 2006. “Mussel Anatomy Lesson Plan”. Accessed November 6, 2020. http://www.museum.state.il.us/ismdepts/zoology/mussels/mussel_anatomy_lesson.html.
Johnston, Jeff. 2020. “CMU Helps an Endangered River Cleaner”. Acessed September 17, 2020. https://www.cmich.edu/news/article/Pages/Snuffbox-mussel-propagation-2020.aspx?utm_source=mes&utm_campaign=our_cmu&utm_medium=email&utm_content=F1-Help+for+a+natural+water+filter.
Michigan Department of Environmental Quality. 2016. “A Biological Survey of the Kalamazoo River Watershed Allegan, Barry, Calhoun, Eaton, Hillsdale, Jackson, Kalamazoo, and Van Buren Counties”. Accessed September 25, 2020. https://www.michigan.gov/documents/deq/wrd-monitoring-report-2014-kalamazoo-watershed_606950_7.pdf.
MichiganDNR. 2020. “Michigan Snuffbox Mussel Propagation | Webber Dam, Ionia County”. Video, October 29, 2020. https://www.youtube.com/watch?v=zMbyX4ToC1s.
Michigan Invasive Species. 2020. “Boating and Fishing Laws to Prevent the Introduction and Spread of Invasive Species. Accessed November 4, 2020. https://www.michigan.gov/invasives/0,5664,7-324-68071_91899—,00.html.
Michigan Natural Features Inventory. 2003. “Surveys of Native Freshwater Mussels in the Lower Reaches of Great Lakes Tributary Rivers in Michigan”. Accessed September 24, 2020. https://mnfi.anr.msu.edu/reports/MNFI-Report-2002-03.pdf.
Modesto, Vanessa et al. 2017. “Fish and mussels: Importance of fish for freshwater mussel conservation”. Fish and Fisheries 1, no. 16: 1-16. https://doi.org/10.1111/faf.12252.
National Geographic. n.d. “Point Source and Nonpoint Sources of Pollution”. Accessed November 4, 2020. https://www.nationalgeographic.org/encyclopedia/point-source-and-nonpoint-sources-pollution/#:~:text=The%20United%20States%20Environmental%20Protection,easily%20identified%20and%20confined%20place.&text=Nonpoint%2Dsource%20pollution%20is%20the,released%20in%20a%20wide%20area.
National Invasive Species Information Center. n.d. “Asian Clam”. Accessed October 30, 2020. https://www.invasivespeciesinfo.gov/aquatic/invertebrates/asian-clam.
Naumann, Robert. 1999. “Corbicula fluminea”. Accessed October 30, 2020. https://animaldiversity.org/accounts/Corbicula_fluminea/.
Perdue University. n.d. “Effect of small population size”. Accessed September 25, 2020. https://www.purdue.edu/captivebreeding/effect-of-small-population-size/.
Piatek, Gary. 2019. “Mussel-strengthening research”. Accessed September 12, 2020. https://www.cmich.edu/news/article/Pages/Kalamazoo-watershed-mussels-research.aspx.
State of Michigan. n.d. “Status and Strategy for Zebra and Quagga Mussel Management”. Accessed September 24, 2020. https://www.michigan.gov/documents/deq/wrd-ais-dreissenids_499881_7.pdf.
Tennessee Wildlife Resource Agency. n.d. “Fresh Water Mussels in Tennessee”. Accessed November 4, 2020. https://www.tn.gov/twra/wildlife/fish/fresh-water-mussels-in-tennessee.html#:~:text=The%20shell%20is%20composed%20of,%2C%20oval%20or%20tear%2Ddrop.
Tip of the Mitt Watershed Council. n.d. “Zebra Mussels”. Accessed September 24, 2020. https://www.watershedcouncil.org/zebra-mussels.html.
Thorp, Ben. 2018. “New research could help ailing Michigan mussels”. Accessed November 5, 2020. https://radio.wcmu.org/post/new-research-could-help-ailing-michigan-mussels#stream/0.
U.S. Fish & Wildlife Service. n.d. “Endangered and Threatened Mussels in the Apalachicola-Chattahoochee-Flint Basin”. Accessed September 25, 2020. https://www.fws.gov/panamacity/resources/ACFmusselsFactSheet.pdf.
Warren, Robert. 2006. “Life Cycle of Freshwater Mussels”. Accessed October 18, 2020. http://www.museum.state.il.us/ismdepts/zoology/mussels/intro_lifecycle.html.
Winhold, Lisa. 2004. “Unionidae”. Accessed September 24, 2020. https://animaldiversity.org/accounts/Unionidae/.
Xerces Society. n.d. “About Freshwater Mussels”. Accessed October 22, 2020. https://xerces.org/endangered-species/freshwater-mussels/about.
